Encapsulation of therapeutic agents in nanoparticles has been a major breakthrough in medicine. It significantly enhances drug delivery by enabling precise targeting, improving bioavailability, and controlling release, thereby minimizing off-target effects and systemic toxicity. It also protects fragile therapeutic agents, like proteins and nucleic acids, from degradation and premature release, ensuring they remain intact until reaching their target. Furthermore, encapsulation improves drug stability, extending shelf life and easing storage and transport. By functionalizing encapsulating materials, targeted therapy becomes possible, directing drugs to specific cells or tissues, particularly valuable in cancer treatment. It can also help overcome drug resistance by delivering drugs directly into cells, bypassing resistance mechanisms, thus revolutionizing medicine with more effective, safe, and targeted drug delivery.
Why Encapsulation efficiency Matters
One essential parameter when it comes to such complexes is encapsulation efficiency. Encapsulation efficiency is a crucial metric that quantifies the success of trapping a therapeutic agent within a delivery system. It represents the percentage of the intended drug that is successfully enclosed within the carrier. This measurement is vital because it directly influences the effectiveness of the drug delivery system, as a higher efficiency translates to a larger proportion of the drug reaching the target site and thus improved therapeutic outcomes. Typically, it’s calculated by comparing the amount of drug successfully encapsulated to the total amount of drug used in the process, serving as a measure of how well the encapsulation procedure performed.
.
Size distribution by taylor dispersion analysis : a powerful alternative to traditional methods
Traditionally a separation between the free therapeutic agent and the encapsulated one is required. This can be done using ultracentrifugation, dialysis or size exclusion chromatography. The concentration of agent will then be compared in both phases, and the ratio of agent in the nanoparticle to free agent will make up the encapsulation efficiency.
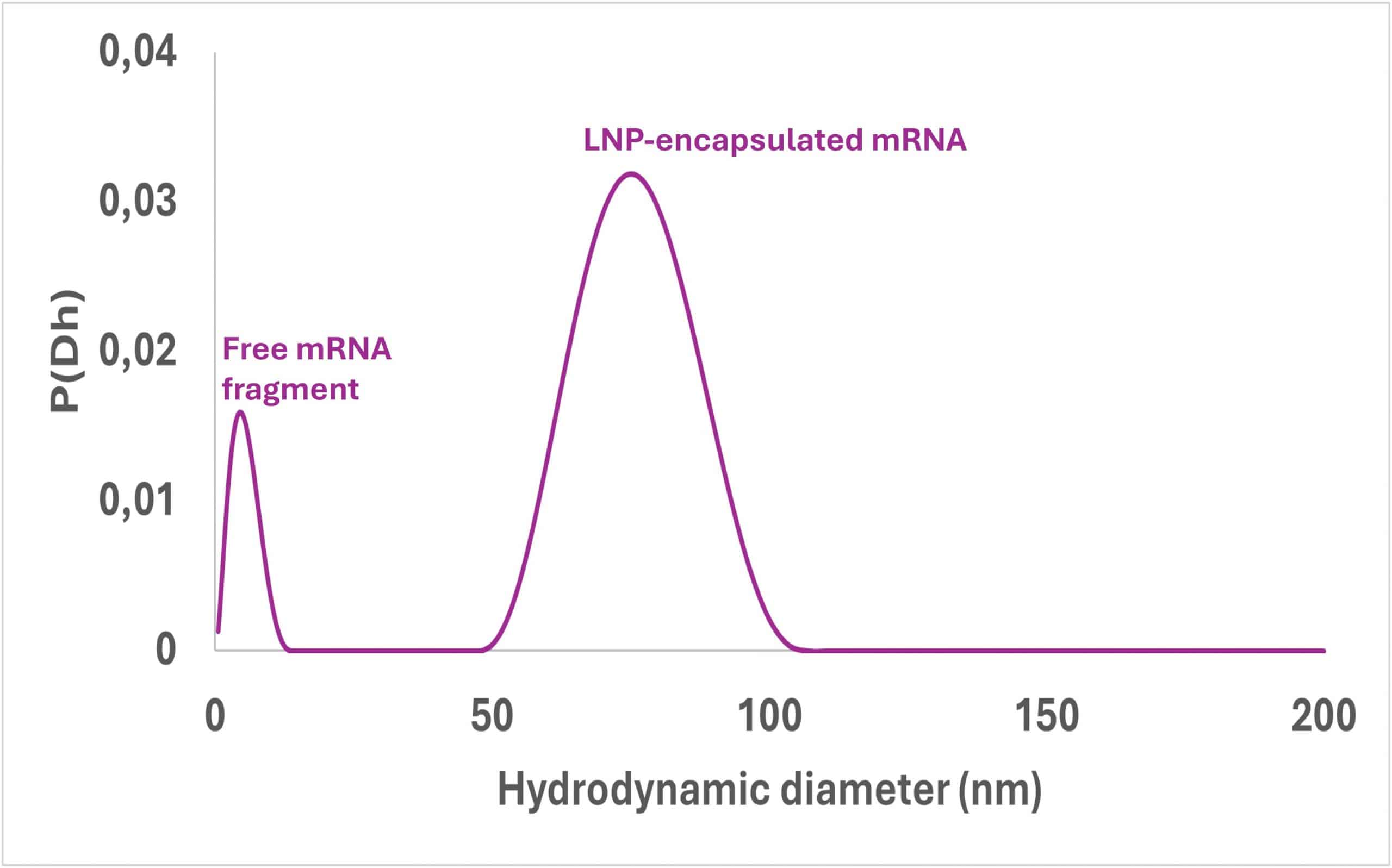
SD-TDA as a size distribution technique is able to determine the size of nanoparticles. However, it is also useful when looking at molecules, RNA or small complexes. Therefore, the size distribution of the different populations in a mixture should provide an accurate representation of the different particles in which the therapeutic agent is located. If the size is molecular the agent will be in free form, if it is the size of the nanoparticle the agent will be encapsulated. The ratio of the area under the curve of each population is the encapsulation efficiency.
This approach can also be used to determine the efficiency of different purification steps, or any analysis that distinguishes between populations in size.
See below an example of Size Distribution obtained by SD-TDA from LNP-encapsulated mRNA
As nanomedicine continues to evolve, Taylor Dispersion Analysis is emerging as a game-changing tool for encapsulation efficiency assessment. By offering high-resolution, label-free, and rapid analysis, SD-TDA enables researchers and pharmaceutical developers to optimize drug delivery systems more efficiently. As an alternative to traditional methods, SD-TDA is paving the way for safer, more effective, and precisely targeted therapeutics.
👉 Want to learn more? Check out our full application note for an in-depth look at the TaylorSizer’s SD-TDA capabilities for LNP characterizations

