Extracellular vesicles (EVs) have captured the attention of the scientific and medical communities for their crucial role in intercellular communication and their potential as diagnostic biomarkers and therapeutic tools. However, studying these tiny particles—especially the smallest ones—has long been a challenge. Enter Size Distribution Taylor Dispersion Analysis (SD-TDA), an innovative technology enhanced by fluorescence detection, as featured in the TaylorSizer by Nanoscale Metrix.
Why conventional Methods fall short?
Traditional methods like ultracentrifugation, Dynamic Light Scattering (DLS), or Nanoparticle Tracking Analysis (NTA) have their merits but also notable limitations:
- Small particles are often missed (<40 nm): These tiny populations remain undetectable.
- Low resolution and aggregation issues: Measurements can be distorted or imprecise.
- Lack of targeted detection: Isolating specific EV populations, like those rich in RNA or proteins, often requires complex labeling workflows.
These challenges have left a knowledge gap in understanding the roles of smaller EVs, which could hold critical molecular insights.
SD-Tda with fluorescence detection: the perfect match
SD-TDA already excels with its ultra-precise size measurement, capable of detecting particles ranging from 0.2 nm to 400 nm. Adding fluorescence detection to the TaylorSizer takes this capability to the next level, unlocking even more potential for sensitive and targeted analysis.
What Makes This Technology Stand Out:
- Unmatched precision: Detect particles as small as 2-5 nm, even at low concentrations, using fluorescence.
- Extended detection range: Intermediate sizes (20-40 nm), often undetectable by NTA, are now fully accessible.
- Targeted detection: Fluorescence allows the isolation of specific populations, such as EVs labeled with fluorescent probes or proteins.
- Minimal sample alteration: While fluorescence boosts sensitivity, it’s not mandatory for all analyses, preserving the sample’s native state.
- Detailed analysis: By combining size and fluorescence data, the TaylorSizer provides a comprehensive view of sample composition and heterogeneity.
Applications that push boundaries
This groundbreaking combination has far-reaching implications for research and applications:
- Diagnostic biomarkers: Ultra-sensitive detection of EVs linked to diseases like cancer or neurodegenerative conditions, even in trace amounts.
- EV-based therapies: Ensure purity, homogeneity, and identity of EVs before clinical trials, including rare subpopulations.
- Basic research: Explore the biological roles of small EVs and associated protein complexes with detailed molecular insights.
- Target-specific detection: Label and track EVs from specific cell types to study their distribution and functions.
Putting it into practice
Studies confirm that SD-TDA with fluorescence complements technologies like NTA or DLS, offering superior sensitivity, resolution, and reproducibility. What’s more, it thrives in complex biological matrices, such as plasma, without requiring excessive or destructive sample preparation.
Fluorescence detection takes things even further:
- Detect specific subpopulations within heterogeneous samples.
- Monitor EVs in real-time within dynamic biological systems.
See below an example of Size Distribution obtained by SD-TDA from secretosome extract
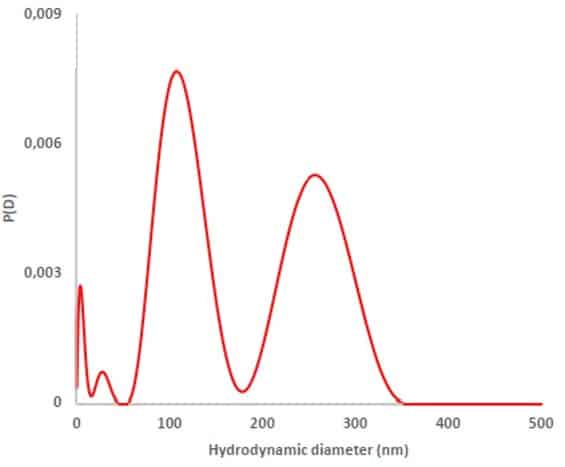
By combining precision, flexibility, and advanced analytical power, SD-TDA with fluorescence is revolutionizing how extracellular vesicles are studied. From basic science to clinical applications, this technology opens up exciting new possibilities in biology, medicine, and biotechnology.
👉 Want to learn more? Check out our full application note for an in-depth look at the TaylorSizer’s SD-TDA capabilities and how it’s transforming EV characterization.